Physician-Directed Nutritional Solutions
Clinical Therapeutic Categories
Post-Viral Syndromes
Medical nutrition targeting documented Long COVID metabolic dysfunction and persistent post-viral fatigue.
Reproductive Disorders
Physician-prescribed nutrition for PCOS, infertility, and documented reproductive metabolic disturbances.
Menopausal Dysfunction
Medical nutrition addressing documented estrogen metabolism disruption and menopausal transition disorders.
Metabolic Syndrome
Advanced medical nutrition for complex insulin resistance and multi-system metabolic dysfunction.
Autoimmune Conditions
Medical nutrition for documented immune dysregulation and inflammatory cascade disorders.
Neurode generative Disorders
Physician-supervised nutrition targeting documented cognitive decline and neurological dysfunction.
Medical Foods

Long COVID Support

Fertility Formula
Medical food providing targeted nutritional support for women with documented metabolic disturbances affecting hormone balance and reproductive function. Contains specialized amino acids, methylation cofactors, and inositols in a physician-supervised formulation.
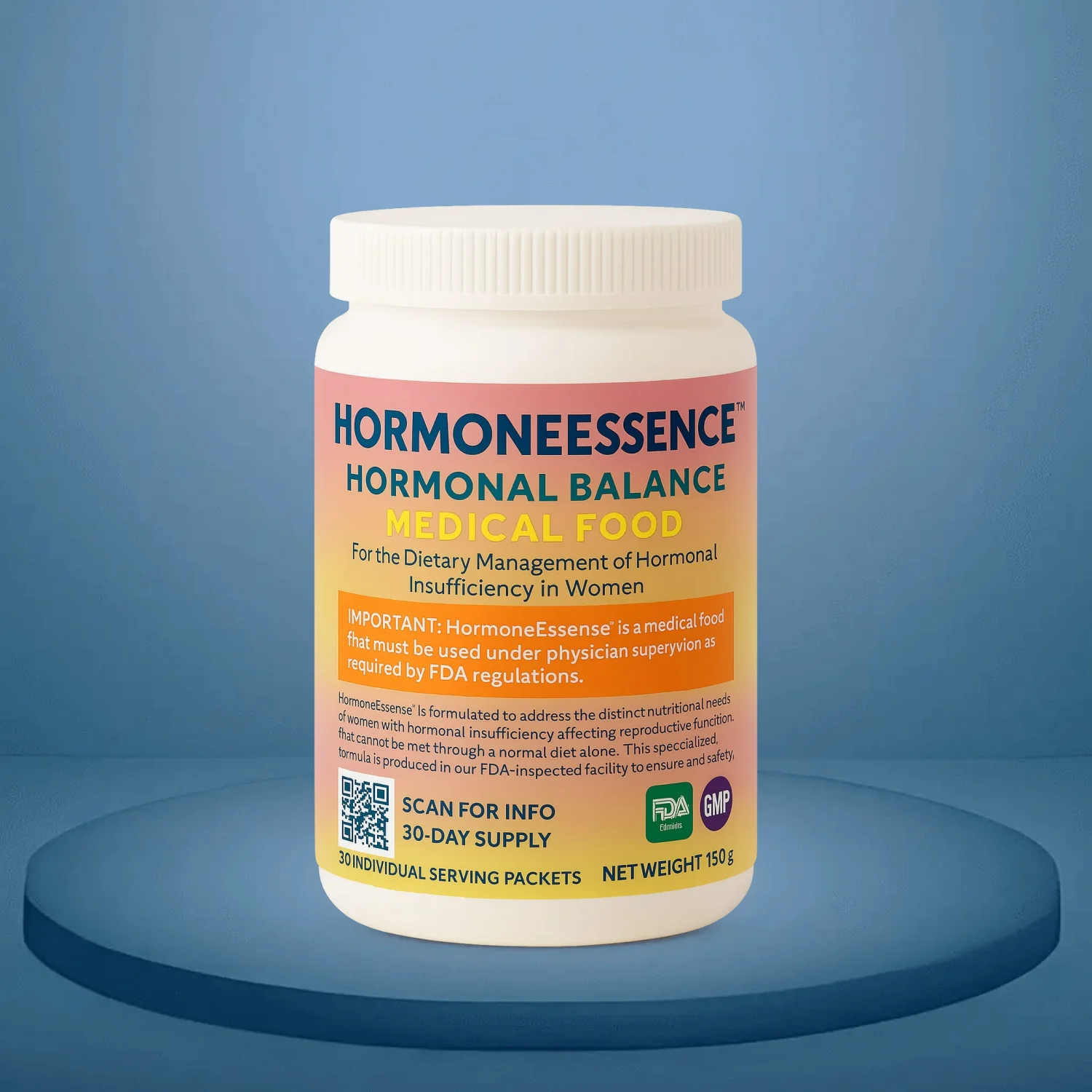
Menopausal Support
Medical food formulated for women with documented metabolic disturbances during perimenopause and menopause, supporting healthy estrogen metabolism, thermoregulation, and bone mineral density. Available only through healthcare practitioners.
Pharmaceutical-Grade Formulation
Metabolic Assessment
Comprehensive biomarker evaluation identifies specific metabolic pathway imbalances.
Facility Location
Targeted nutritional compounds selected based on objective biomarker data.
NSF Registration
Produced in certified cGMP facilities under strict quality control.
Standardized Potency
Each formulation contains precisely quantified nutritional compounds with verified bioactivity
Manufacturing Excellence Partnership
Pharmaceutical-Grade Medical Food Development Under Licensed Medical Oversight
Licensed Healthcare Provider Integration
Prescription-Based Medical Food Development
Key Compliance Elements:
Licensed Practitioner Oversight
All medical foods require valid prescriptions from licensed healthcare providers.
Medical Necessity Documentation
Each formulation addresses specific medical conditions with distinctive nutritional requirements.
Prescription Verification Process
Comprehensive validation system ensures all prescriptions meet regulatory standards.
Healthcare Provider Network
Established relationships with licensed practitioners across multiple jurisdictions
NSF GMP Registered Manufacturing Excellence
Freedom to Formulate Facility Credentials
NSF GMP Registration


FDA Compliance
Licensed Healthcare Provider Network


HIPAA Compliance
Precision Manufacturing
Patient Safety & Regulatory Compliance Framework
Comprehensive Medical Oversight Process
Professional Prescription Documentation System
Sample Medical Food Prescription Process
Innovation1 Bio utilizes Freedom to Formulate's established prescription documentation system, which has been refined over 10+ years of serving healthcare practitioners. The standardized prescription form demonstrates our commitment to proper medical oversight and regulatory compliance.
Digital Prescription Processing
Freedom to Formulate's cloud-based digital formulator platform enables healthcare practitioners to create, submit, and track prescriptions through a secure, HIPAA-compliant system. This advanced technology ensures prescription accuracy while maintaining complete documentation for regulatory compliance and patient safety monitoring.
Prescription Authorization Process
Licensed Practitioner Assessment
Healthcare providers evaluate patients for medical conditions requiring specialized nutritional support.
Medical Necessity Documentation
Comprehensive clinical documentation establishing distinctive nutritional requirements.
Prescription Issuance
Valid prescriptions issued by licensed healthcare providers for specific medical foods.
Prescription Verification
Multi-step validation process ensuring prescription authenticity and regulatory compliance
Quality Assurance Protocols
- Raw Material Qualification– Identity, purity, and potency verification for all ingredients
- In-Process Testing– Multiple quality checkpoints throughout manufacturing
- Finished Product Analysis– Comprehensive testing for potency, purity, and safety
- Batch Documentation– Complete traceability records for regulatory compliance
- Stability Testing– Shelf-life validation and efficacy maintenance verification
Patient Safety Measures
- Adverse Event Reporting– Established protocols for monitoring and reporting patient responses
- Healthcare Provider Communication– Direct channels for practitioners to address patient concerns
- Contraindication Screening– Comprehensive evaluation of potential drug-nutrient interactions
- Follow-Up Protocols– Systematic patient monitoring through healthcare provider network
HIPAA-Compliant Patient Services
Protected Health Information Management
Secure Patient Portals
Encrypted communication channels for healthcare providers and patients.
PHI Protection Protocols
Comprehensive data security measures exceeding federal requirements.
Access Controls
Restricted access to patient information on need-to-know basis.
Audit Trails
Complete documentation of all PHI access and usage.
Business Associate Agreements
Formal HIPAA compliance agreements with all partners.
Licensed Practitioner Network
Medical Professional Oversight Structure
Medical Director
Board-certified physician providing clinical oversight of all medical food prescriptions.
Licensed Practitioners
Network of physicians, nurse practitioners, and healthcare providers authorized to prescribe medical foods in their respective jurisdictions
Clinical Assessment Team
Licensed professionals specializing in metabolic health and nutritional medicine.
Regulatory Compliance Officer
Dedicated personnel ensuring adherence to all federal and state regulations
Manufacturing Partner Transparency
Freedom to Formulate Facility Details
Licensed Practitioner Assessment
Personalized & Compounded Nutrients, LLC (dba Freedom to Formulate).
Facility Location
Sisters, Oregon.
NSF Registration
GMP Registered Facility.
Experience
10+ years serving healthcare practitioners.
Specialization
Physician-directed medical food compounding.
Regulatory Status
FDA-compliant medical food manufacturing.
Advanced Digital Formulation System
- Cloud-based practitioner formulator platform
- Real-time prescription processing and validation
- Individual patient-specific formulations
- 450+ high-quality ingredient options
- Automated compliance checking protocols
Manufacturing Capabilities
- Made-to-order custom medical food development
- Small-batch precision compounding (low minimum orders)
- Individual patient-specific formulations
- Triple-verification quality control testing
- 100% excipient/chemical filler-free options
- Temperature-controlled storage and distribution
- Independent third-party laboratory testing
Regulatory Compliance Documentation
Comprehensive Legal Framework
Federal Compliance
- FDA Medical Food Regulations (21 CFR 101.9(j)(8))
- HIPAA Protected Health Information Requirements
- Federal Trade Commission Guidelines
- DEA Controlled Substance Regulations (where applicable)
State Compliance
- Licensed healthcare provider requirements in all operating jurisdictions
- State pharmacy and compounding regulations
- Professional licensing verification
- Interstate commerce compliance
International Compliance
- International operations compliance (Israel facility).
- Cross-border prescription validation.
- International patient privacy regulations.
Continuous Monitoring & Quality Assurance
Ongoing Compliance Verification
Regular Facility Audits
Quarterly inspections of manufacturing processes.
Prescription Compliance Reviews
Ongoing validation of practitioner prescribing patterns.
Patient Safety Monitoring
Systematic tracking of patient outcomes and adverse events.
Regulatory Updates
Proactive monitoring of changing regulations and compliance requirements.
Staff Training Programs
Continuous education for all personnel handling medical foods
Patient Safety Commitment
Contact Information
Freedom to Formulate (Manufacturing Partner)
NSF GMP Registered Facility

